Home » Inorganic Fine Chemicals and Metal Salts » Silver Sulfate
What is Silver Sulfate
Silver Sulfate is a white to gray solid powder. It is an inorganic chemical compound that is completely odorless and only slightly soluble in water. It is stable under normal conditions of use and storage, though it turns darker when it is exposed to air or light.
| PRODUCT SPECIFICATIONS | |
|---|---|
| Name of Product | Silver Sulfate |
| CAS NO | 10294-26-5 |
| Synonyms | Silver sulphate; Disilver sulfate; Argentous sulfate; sulfato de prata; sulfato de plata; Argentum sulfate; Sulfate d'argent |
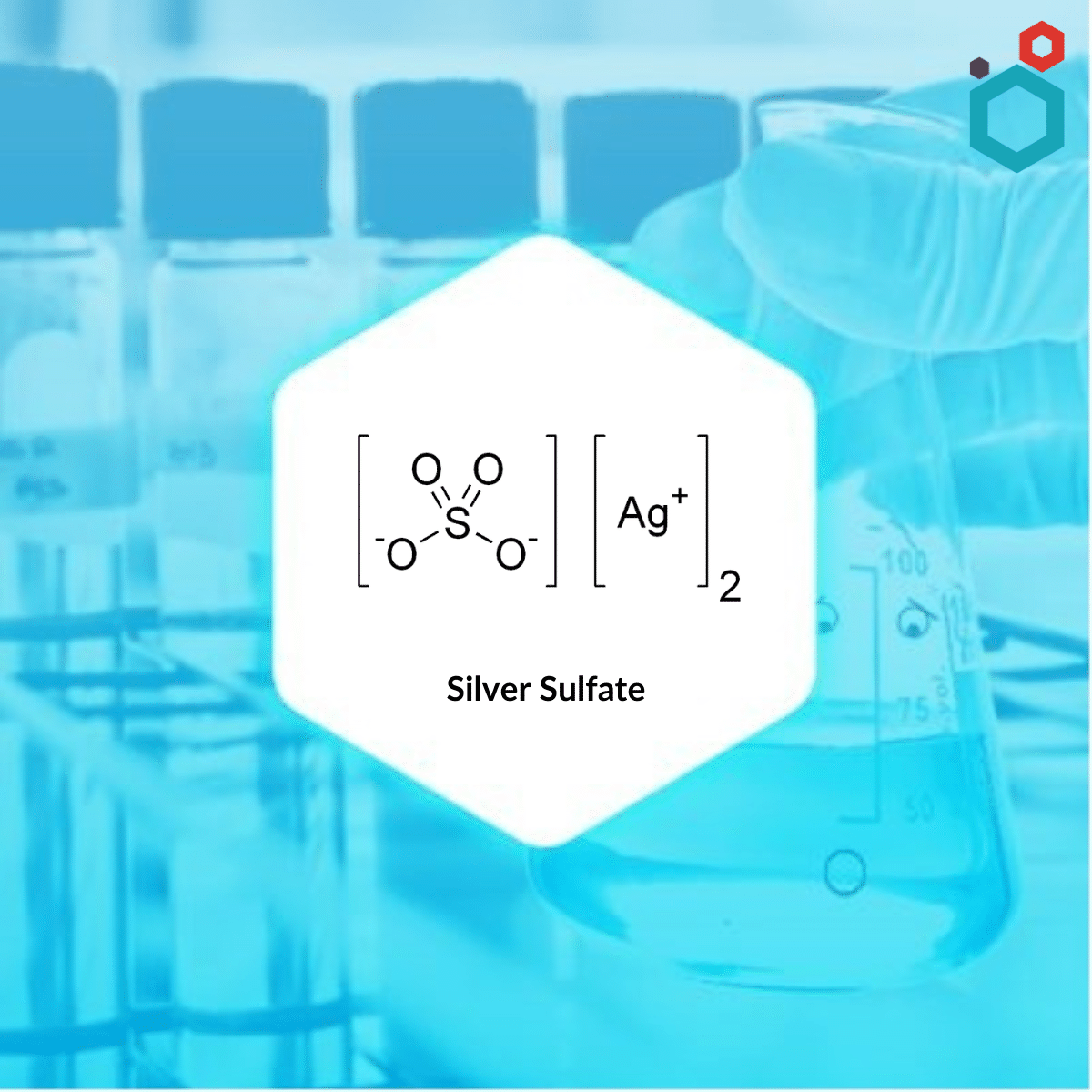
| MOLECULAR FORMULA | Ag2SO4 |
| Molecular Weight | 311.80 g/mol |
| USES | Mainly used for medicinal purposes as antibacterial agent |
| Pubchem CID | 159865 |
| Pubchem SID | 462770302 |
TECHNICAL SPECIFICATIONS
| SR. No | Criteria | Limit/Specification |
|---|---|---|
| 1 | Appearance (Color) | white |
| 2 | Appearance (Form) Powder and/or Lumps | Conforms to Requirements |
| 3 | Titration by KSCN % Ag | ≥ 68.1 % |
| 4 | X-Ray Diffraction | Conforms to Structure |
| 5 | Iron (Fe) | ≤ 0.001 % |
| 6 | Insoluble matter and Silver Ch | ≤ 0.02 % |
| 7 | Nitrate (NO3) | ≤ 0.001 % |
| 8 | Substances not ppt. by HCl | ≤ 0.03 % |
| 9 | Trace Metal Analysis | ≤ 100.0 ppm |
| 10 | Purity >= 99.99% Based on Trace Metal Analysis | Must Meets Requirements |
Uses
- Silver sulfate is mostly used in the silver plating of objects and in electrolysis.
- For medicinal purposes, it is used as an antimicrobial agent. Silver compounds, especially silver sulfate, are used to saturate bandages used to cover a variety of skin wounds, from minor cuts to severe lacerations and abrasions.
- The combination of silver sulfate and iodine is an effective reagent for iodinating alkyl, alkoxybenzenes, aromatic amines, and uridines.
- It serves as a non-staining alternative to silver nitrate.
- It is also utilized in the production of batteries.
FAQs
Q. Is Silver Sulfate soluble in water?
Silver sulfate has low solubility in water, i.e. approximately about 0.83 g/100 mL at 25 °C.
Q. What is the chemical formula for Silver Sulfate?
The chemical formula for silver sulfate is Ag2SO4.
Q. Is Silver Sulfate a precipitate?
When an aqueous solution of silver nitrate is reacted with sulfuric acid, silver sulfate separates out as a solid precipitate.
2 AgNO3 + H2SO4 → Ag2SO4 + 2 HNO3
