Home » Blog » Malachite Green »
Table of Contents
What is Malachite Green
Malachite Green is an organic monochloride salt of the malachite green cation. Although the name of the compound is malachite green it is not prepared from the malachite mineral and the name has been associated with it because of its intense green colour. Malachite green, also known as aniline green, benzaldehyde green, or china green, is a triphenylmethane dye and is used as a local antiseptic in a dilute solution. Fungi and gram-positive bacteria are resistant to malachite green. It is also known to control the fungus Saprolegnia, a water mould that destroys eggs and young fishes in the fish breeding industry.
Malachite green is also used to dye cotton that has been mordanted with tannin and as a direct dye for silk, wool, jute, and leather. The dye is a lustrous green crystal that is soluble in water and alcohol and is made from benzaldehyde and dimethylaniline.
Chemical Structure & Properties of Malachite Green
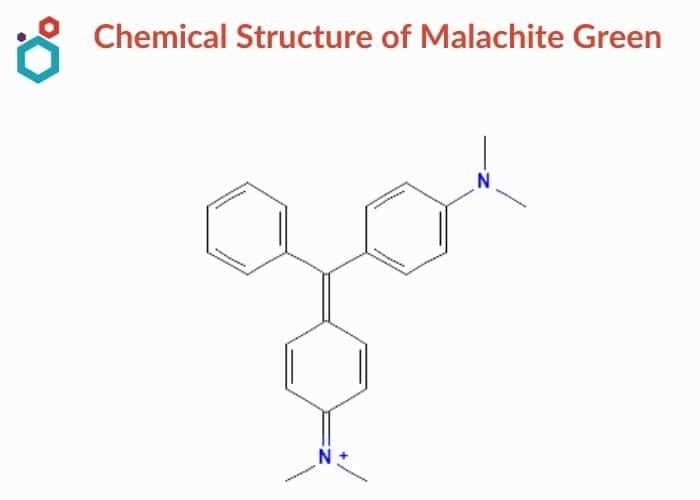
- Malachite green is a triarylmethane dye used in the dyestuff industry and in the pigment industry with the chemical formula of C23H25ClN2.
- Malachite green refers to the chloride salt [C6H5C(C6H4N(CH3)2)2]Cl in scientific jargon. The oxalate salt, in addition to the chloride salt, is also available in the market.
- Malachite green is a phrase that is widely used to describe a coloured green cation. The colour of the compound appears to be unaffected by the anions. Normally, the cation’s bright green colours are caused by a high absorption band at 621 nm wavelength.
- The condensation of benzaldehyde and dimethylaniline yields leuco malachite green (LMG), which is used to make malachite green.
C6H5CHO + 2 C6H5N(CH3)2 → C6H5CH(C6H4N(CH3)2)2 + H2O - The colourless leuco product, which is related to triphenylmethane, is further oxidised to a cation, Malachite Green. The following is the reaction:
C6H5CH(C6H4N(CH3)2)2 + HCl + 1⁄2 O2 → [C6H5C(C6H4N(CH3)2)2]Cl + H2O - Malachite green (MG) on hydrolysis produces the following alcohol:
[C6H5C(C6H4N(CH3)2)2]Cl + H2O → C6H5C(OH)(C6H4N(CH3)2)2 + HCl
The alcohol formed here is crucial because it promotes the passage of malachite green across cell membranes. It’s usually converted into leuco malachite green once it’s within the cells. - It’s important to realise that only the malachite green compound variant is a deep green colour. The leuco and alcohol malachite green derivatives, on the other hand, are not. This is owing to the fact that the compound’s cationic form extends a pi-delocalization, which causes the molecule to absorb visible light.
Uses of Malachite Green
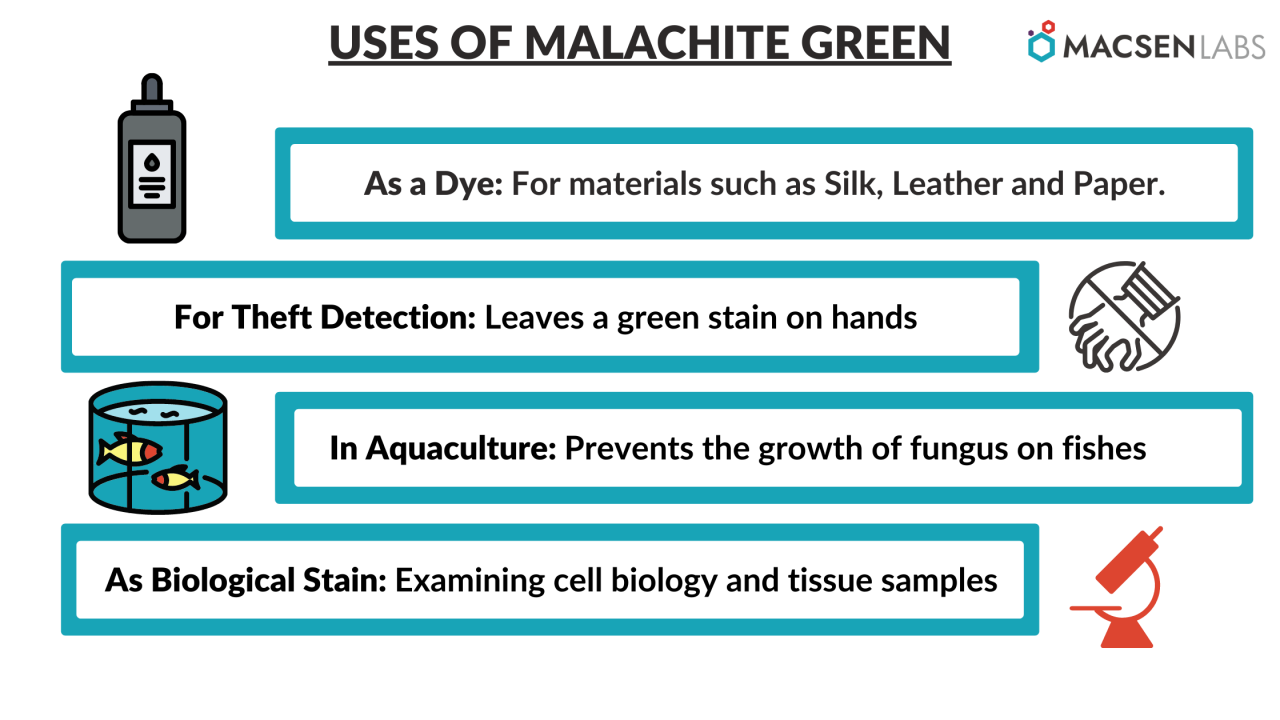
- As a Dye – Malachite green has been used as a dye for centuries. For this purpose, kilotonnes of MG and related triarylmethane dyes are produced each year.
- Treatment of Saprolegnia – Malachite Green has been used to treat Saprolegnia and as an antibacterial. It is active against the oomycete Saprolegnia, which infects fish eggs in commercial aquaculture. In freshwater aquariums, it is a popular treatment for Ichthyophthirius multifiliis.
- For theft detection – Thieves and pilferers have frequently been apprehended using Malachite Green. The anhydrous powder is put on the bait, which is usually money. Anyone handling contaminated money will notice that washing their hands will leave a green stain on their skin that will last for several days.
- As a biological stain – Malachite Green is also used as a biological stain for examining cell biology and tissue samples under a microscope. Basic fuchsin stains bacteria magenta or red, and MG is used as a blue-green counterstain in the Gimenez staining method. Because MG can directly stain endospores within bacterial cells, it is also used in endospore staining; however, a safranin counterstain is frequently used. Alexander’s pollen stain includes MG. MG is used as a saturable absorber in dye lasers or as a pH indicator with a pH range of 0.2 to 1.8. However, this is a relatively uncommon application. In forensic science, leuco-malachite green (LMG) is used as a latent blood detection method. The reaction between LMG and hydrogen peroxide is catalyzed by haemoglobin, which turns the colourless LMG into MG. As a result, the presence of blood is indicated by the appearance of green colour.
- In Aquaculture – Malachite Green is also effective at preventing fungus from growing on fish eggs. MG can help with the following ailments:
-
- Egg Fungus – Protects fish eggs by acting as a disinfectant.
- Ichthyophthirius multi-files – Fish scratches against objects, small white spots resembling sand.
- Oödinium pillars– Also known as Velvet, are the result of a parasitic infection.
- Saprolegnia – A type of freshwater mould that eats fish eggs.
Does Malachite Green treat Fin Rot?
Fin rot is a very common contagious disease in fish & it is a bacterial infection characterized by torn & ragged fins. Your fish infected with fin rot may seem discoloured & lazy. If your fish having fin rot are not treated well, then your fish’s fins might get permanent damage & it could prove deadly. Malachite Green can be used to treat Fin Rot but it is not the primary agent of choice because of its ability to cause stress to the aquarium inhabitants if ingested.
How to use malachite green in aquaculture
Shake well before using, according to the manufacturer’s instructions. Remove all carbon from the filter and discard it, but do not turn off the filtration system. Typically cures ich in 24 hours; if necessary, treatment can be repeated in 24-48 hours. If the fish are stressed, change half of the water. The time could be up to 10 days to completely irradiate an infestation.
To reduce toxicity, carbon or other similar chemical filter media can be replaced after each treatment or temporarily added to the filter for 2 hours prior to each subsequent treatment before being removed again (correct mineralization and a pH above 7 are also important). Quick Cure turns the water blue for a short time.
- 1 mL is enough to treat 18 gallons (68 litres)
- 1 teaspoon is enough to treat 90 gallons of water (340 litres)
- 1 capful is enough to treat 250 gallons of water (950 litres)
- 500 gallons per 1 fluid oz (1900 litres)
Side effects
Toxicity: Exposure time, temperature, and concentration all increase the dye’s toxicity. Mutagenesis, chromosomal fractures, Carcinogenesis, teratogenicity, and respiratory toxicity have all been reported. Multi-organ tissue injury is one of the histopathological effects of MG. In MG-exposed fish, biochemical parameters in the blood undergo significant changes. Kidney, muscles, serum, liver, and other tissues, as well as eggs and fry, have been found to contain MG and its reduced form, leucomalachite green. Organ damage, mutagenic, carcinogenic, and developmental abnormalities are all symptoms of toxicity in some mammals.
FAQs
Q. Is malachite green toxic to humans?
Yes, malachite green is toxic to humans. Carcinogenesis, mutagenesis, chromosomal fractures, teratogenicity, and pulmonary toxicity have all been described as symptoms caused due to toxicity from the consumption of Malachite Green.
Q. What is malachite green soluble in?
Malachite Green is a glossy green crystal that is soluble in water and alcohol and is made from benzaldehyde and dimethylaniline.
Q. How fast does malachite green work?
Typically cures ich in 24 hours; if required, medication can be repeated in 24-48 hours. If the fish are stressed, change half of the water. It might take up to ten days to completely irradiate an infestation.
Disclaimer-
The information provided here is based on general knowledge, articles, research publications etc. We do not claim the authenticity of any of the information provided above. We do not claim or suggest/advise any medical, therapeutic, health or nutritional benefits of Malachite Green. We do not supply or promote our Malachite Green product for the applications which are covered by valid patents and which are not approved by the FDA.
Macsen Labs is a manufacturer and supplier of several grades of Malachite Green such as:-

