Table of Contents
What are Biological Stains?
Biological stains, often known as histological stains or simply stains, are chemical substances that are utilized in biological and medical laboratories to improve the visibility of particular structures, cells, or tissues when viewed via a microscope. These stains function by preferentially binding to certain cellular components or structures. This gives the specimen a color and makes it easier for researchers, pathologists, and other medical professionals to examine and identify different cellular parts.
Biological Stains are frequently used in a wide variety of applications in the field of Microbiology, Hematology, Histology, Cytology, Parasitology, Genetics and Molecular Biology, Protein and DNA staining after Electrophoresis, Fluorescence Microscopy, Botany and Plant Biology, Aquatic Biology, etc.
Classification of Biological Stains
Biological stains can be classified based on various criteria, including their origin, chemical properties, target structures or substances, mechanism of action, and applications. Some common classification categories for biological stains are as follows-
On the basis of Origin
- Natural dyes: These dyes or colorants have a natural origin and are derived from plants, invertebrates, or minerals. Examples include Hematoxylin.
- Synthetic dyes: These dyes are manufactured synthetically. Examples include Methylene blue.
On the basis of Chemical Composition
- Basic dyes: These dyes have a positive charge and a pH above 7, primarily having an affinity for acidic tissue components (acidophilic). Examples include Crystal violet, Methylene blue, and Safranin.
- Acidic dyes: These dyes have a negative charge and a pH below 7, primarily having an affinity for basic tissue components (basophilic). Examples include Eosin and Acid fuchsin.
- Neutral dyes: These dyes have both acidic and basic properties and a pH equal to 7, can be used in a wide range of staining applications.
On the basis of Solubility
- Water-soluble stains: These stains dissolve easily in water and are commonly used in routine histology and cytology procedures.
- Lipid-soluble stains: These stains dissolve in lipids and are used to detect lipid components in cells or tissues.
On the basis of Mechanism of Action
- Absorption dyes: These dyes functions depending on both the charge of the dye and the tissue.
- Direct staining dyes: These dyes are differentially absorbed into the tissue.
- Indirect staining dyes: The tissue is over-stained and then a process called differentiation is used to remove the excess stain.
- Mordant dyes: These dyes have chelating sites to form stable coordination complex with metal ions from metal salts (mordants). Dyes can form chelates with different mordants to develop various shades.
On the basis of Fluorescence properties
- Fluorescent stains: These stains emit light when exposed to specific wavelengths and are used in fluorescence microscopy and other fluorescence-based techniques. Examples include Fluorescein, Eosin Y.
- Non-fluorescent stains: These stains provide color contrast without emitting light. Examples include Giemsa Stain.
Examples of Biological Stains
Acriflavine
Acriflavine is an organic compound that belongs to the family of dyes known as acridines. It is also known by its chemical names “3,6-Diamino-10-methylacridinium chloride” or “3,6-Diaminoacridine hydrochloride”. Acriflavine is a synthetic dye with a yellow or yellow-brown color and was first synthesized in the late 19th century.
It has been historically used for various applications, including as a biological stain, an antiseptic, and an antimicrobial agent. It is also used to treat external fungal infections in aquarium fish, for the disinfection of fish eggs, treatment of open wounds, and treatment of external protozoan infections.

Auramine O
Auramine O is a synthetic fluorescent dye belonging to the family of aniline dyes. Its chemical name is “4-[4-(dimethylamino)benzenecarboximidoyl]-N,N-dimethylaniline;hydrochloride”. It is a diarylmethane dye and functions as both a fluorochrome and a histology stain.
It is primarily used as a fluorescent stain in microbiology for the detection of acid-fast bacteria, especially Mycobacterium species. It is also used for the detection of other fungi and parasites in histological samples.

Brilliant Green
Brilliant Green is a synthetic dye belonging to the family of triphenylmethane dyes. Its chemical name is “ethyl [4-4-[ethyl[3-(4-sulfobenzyl)thiazol-2-yl]amino]phenylthiazol-2-yl]azanium”. It is commonly used as a biological stain, an antiseptic, and a selective bacterial growth inhibitor.
It is used as a biological stain in microbiology to differentiate and identify certain bacterial species. It is part of the selective agar medium known as Brilliant Green Agar (BGA), which inhibits the growth of most gram-negative bacteria while allowing the growth of specific gram-negative bacteria, such as Salmonella and some Shigella species.

Crystal Violet/Gentian Violet
Crystal Violet, also known as Gentian Violet or Methyl Violet 10B, is a synthetic triarylmethane dye. Its chemical name is “3,7-bis(dimethylamino)phenazathionium chloride”. It has various well-known applications in microbiology, histology, and other scientific fields.
It is widely used as a biological stain in microbiology, particularly in Gram staining. It is a vital stain that can be used on living tissues or cells to visualize specific structures or assess cell viability. In histology, Crystal Violet is used in combination with other stains, such as Safranin, to stain plant tissues, especially for the visualization of plant cell walls.
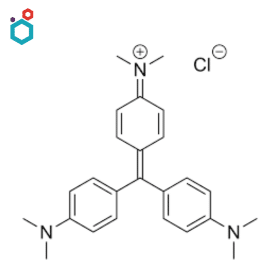
Resources: Gentian Violet | Crystal Violet | Properties, Uses and Side Effects
Eosin Y
Eosin Y is a synthetic dye belonging to the family of xanthene dyes. Its chemical name is “2′,4′,5′,7′-Tetrabromofluorescein”. It is commonly used as a biological stain in histology and pathology. It is an acidic dye that binds to basic or positively charged cellular structures.
It is used for staining tissues and cells to aid in their visualization and examination under a microscope. It is often used as a counterstain in combination with other dyes, such as hematoxylin, in staining techniques like Hematoxylin and Eosin (H&E) staining. It is also used to stain tissue sections and highlight cellular components, such as cytoplasm, connective tissue fibers, and extracellular structures.

Resources: Eosin | Properties, Available variants & Uses
Erythrosine B
Erythrosine B, also known as Red No. 3, is a synthetic cherry-pink or red food dye. Its chemical name is “2′,4′,5′,7′-Tetraiodofluorescein”. It is commonly used as a food coloring agent to add a bright red or pink color to a variety of food and beverage products.
Erythrosine is sometimes used as a counterstain in histology and cytology procedures to provide contrast and enhance visualization. It is particularly useful in conjunction with other stains, such as hematoxylin, for visualizing proteins, connective tissues, fibres, and keratin in sample material of human origin.
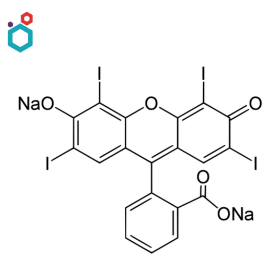
Fluorescein
Fluorescein, also known as Uranine or Acid Yellow 73, is a synthetic dye belonging to the family of xanthene dyes. It is a highly fluorescent dye that comes in the form of a dark orange/red powder that is only slightly soluble in water and alcohol.
It is commonly used as a biological stain in scientific and medical research. As a fluorescent dye, fluorescein has the ability to bind to specific cellular structures, proteins, or other biomolecules and emit bright green fluorescence when exposed to light of a specific wavelength and is thus used in fluorescence microscopy to label and visualize cellular components in living or fixed cells and tissues.

Resources: Fluorescein & its Sodium Salt | Chemistry, Uses and Side effects
Giemsa Stain
Giemsa stain, also known as Wright-Giemsa stain, is a combination stain widely used in cytology, histology, and hematology for staining blood smears, bone marrow samples, and other biological specimens. It is named after Gustav Giemsa, a German chemist who developed the stain in the late 19th century.
It is a differential stain that includes a combination of eosin dye, methylene blue, and azure in its composition. It is used in cytology to stain cells in body fluids, such as pleural or peritoneal fluid, for examination. In histology, Giemsa stain is used to stain tissue sections for the identification and characterization of cellular structures and components.
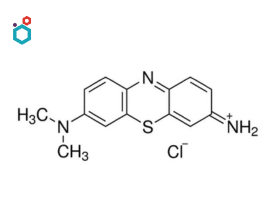
Resources: Giemsa Stain | Composition, Principle, Procedure & Uses
Hematoxylin
Hematoxylin or Haematoxylin, also called natural black 1, is a natural dye extracted from the heartwood of certain trees, such as the logwood tree (Haematoxylum campechianum). It is a basic dye that binds to acidic components in cells, especially nucleic acids like DNA and is one of the most commonly used biological stains in histology and pathology.
It is often used in combination with eosin in a staining technique known as Hematoxylin and Eosin (H&E) staining, one of the most widely used staining methods in histopathology. It is also a part of the Papanicolaou stain, often known as PAP stain, which is used to examine cytology specimens.
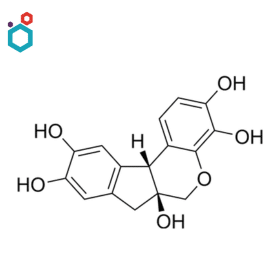
Leishman’s Stain
Leishman stain, also known as Leishman’s stain, is a type of the Romanowsky stain. It is a combination stain that involves mixing two dyes: Eosin Y and Methylene Blue. The stain is named after William Boog Leishman, a British pathologist who developed the technique in the late 19th century.
It is a biological stain commonly used in laboratory settings, especially in the field of hematology to separate and identify distinct types of leukocytes (white blood cells) and in parasitology for malaria parasite identification. Its primary use includes staining of blood smears to examine blood cells and identify various cellular elements.
Lissamine Green
Lissamine Green is a synthetic dye belonging to the family of xanthene dyes. Its chemical name is “4-(4-dimethylaminophenylazo)-2,5-disulfonatephenol”. It is commonly used as a biological stain and dye in various medical and research applications.
It is primarily used in ophthalmology and optometry to stain the surface of the eye and highlight specific ocular structures, in corneal staining and in the assessment of dry eye conditions. It is a vital stain that can be used on living tissues or cells to visualize specific structures or assess tissue health.

Malachite Green
Malachite Green is a synthetic dye belonging to the family of triarylmethane dyes. Its chemical name is “4-[bis(4-dimethylaminophenyl)methylene]-N,N-dimethylbenzenamine”. It is a versatile dye with various applications in different fields, including biology, microbiology, and aquaculture.
It is commonly used as a biological stain in laboratory settings. Being a basic (cationic) dye, it binds to negatively charged cellular structures, such as nucleic acids (DNA and RNA) and some proteins, making it useful for staining cell nuclei and differentiating them from the surrounding cytoplasm in tissues and cells. It is also used to stain specific microorganisms, such as spores of certain bacteria and fungi.
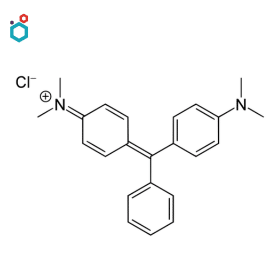
Resources: Malachite Green | Properties, Uses, Side Effects & Application in Aquaculture
Methylene Blue
Methylene Blue or Basic Blue 9 is a synthetic dye belonging to the family of thiazine dyes. Its chemical name is “3,7-bis(dimethylamino)phenazathionium chloride”. It is a type of dye that sees widespread applications in various fields, including biology, medicine, chemistry, and microscopy.
It is a popular stain used for a variety of activities, including bacterial identification and cellular structure in both plant cells and animal cells, identification of micro-organisms, identifying nuclei acids and RNA sequences, distinction between bacterial, viral & fungal diseases, determining cell viability in yeast samples, etc.

Resources: Methylene Blue Dye | Uses & Side effects
Methylene Blue as a Stain | What is it used for with Example
Patent Blue V
Patent Blue V, also known as Food Blue 5, is a synthetic dye belonging to the family of triarylmethane dyes. Its chemical name is “sodium [4-4-(ethyl[(3-sulfonatophenyl)methyl]amino)phenylmethyl]benzenesulfonate”. It occurs as dark blue powder or granules. It is primarily used as a blue food dye to add a blue color to various food and beverage products.
In medicine and surgery, Patent Blue V is used as a vital stain for identifying lymphatic vessels and lymph nodes during surgical procedures. It is particularly useful in sentinel lymph node mapping, which provides critical information for cancer staging and treatment planning.

Rhodamine B
Rhodamine B is a synthetic fluorescent dye belonging to the family of xanthene dyes. Its chemical name is “9-(2-carboxyphenyl)-3,6-bis(diethylamino)xanthylium chloride”. It is widely used as a biological stain and a fluorescent dye in various scientific and medical applications.
As a biological stain, it is used to label and visualize cellular structures, proteins, and other biomolecules in living or fixed cells and tissues. It is utilized to a significant extent in biotechnology applications such as fluorescence microscopy, flow cytometry, fluorescence correlation spectroscopy and ELISA. It is also used in combination with Auramine O as auramine-rhodamine stain to stain acid-fast organisms, e.g. Mycobacterium.
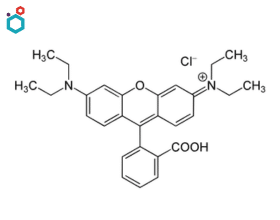
Rose Bengal
Rose Bengal is a synthetic dye belonging to the family of xanthene dyes. Its chemical name is “4,5,6,7-tetrachloro-2′,4′,5′,7′-tetraiodofluorescein”. It is a versatile dye that has various applications in different fields, including biology, medicine, and photography.
It is used as a biological stain (vital stain) in microbiology and histology to stain certain cellular structures and organisms, such as bacteria, yeast, and other fungi. The stain is particularly useful for differentiating live from dead cells, as it is taken up more readily by damaged or dying cells. It is also used as a diagnostic agent for certain eye conditions in ophthalmology.

Safranin
Safranin, also known as Safranin O or Basic Red 2, is a synthetic lipophilic cationic dye belonging to the family of phenazine dyes. Its chemical name is “4-[bis(4-dimethylaminophenyl)methylene]-2,5-cyclohexadien-1-iminium chloride”. It has various applications in biology, histology, and microbiology.
It is widely used as a biological stain in laboratory settings. It is used as a counterstain in Gram staining (a common technique for classifying bacteria into Gram-positive and Gram-negative groups based on their cell wall composition) and in various other staining protocols such as Safranin-O and Fast Green stain ( commonly used for staining plant tissues).

Wright Stain
Wright’s stain, also known as the Wright-Giemsa stain, is a modification of the original Romanowsky stain, developed independently by James Homer Wright in the United States and Gustav Giemsa in Germany. The stain combines two dyes: Eosin Y, an acidic dye, and Methylene Blue, a basic dye. The combination of these dyes produces differential staining, imparting different colors to various cellular components, enabling the identification of different cell types and structures.
It is commonly used in hematology and cytology for staining blood smears aiding in the diagnosis and classification of different blood disorders and infections. It is also used for the purpose of staining chromosomes, bone marrow aspirates, and urine samples to identify Urinary Tract Infections (UTIs).
Other Biological Stains
- Azure B
- Azure II Eosinate
- Basic Brown 1 (Bismarck Brown Y)
- Basic Fuchsin
- Basic Violet 2 (New Fuchsin)
- Leucocrystal Violet
- Methyl Violet
- Pararosanline
- Phloxine B
- Proflavine Hemisulphate
- Trypan Blue
Disclaimer-
The information provided here is based on general knowledge, articles, research publications etc. and we do not claim the authenticity of any of the information provided above. We do not claim or suggest/advise any medical, therapeutic, health or nutritional benefits of our Biological Stains. We do not supply or promote our Biological Stains for the applications which are covered by valid patents and which are not approved by the FDA.
Macsen Labs is a manufacturer and supplier of a range of Specialty Dyes and Biological Stains.

