What is Dimethylglyoxime?
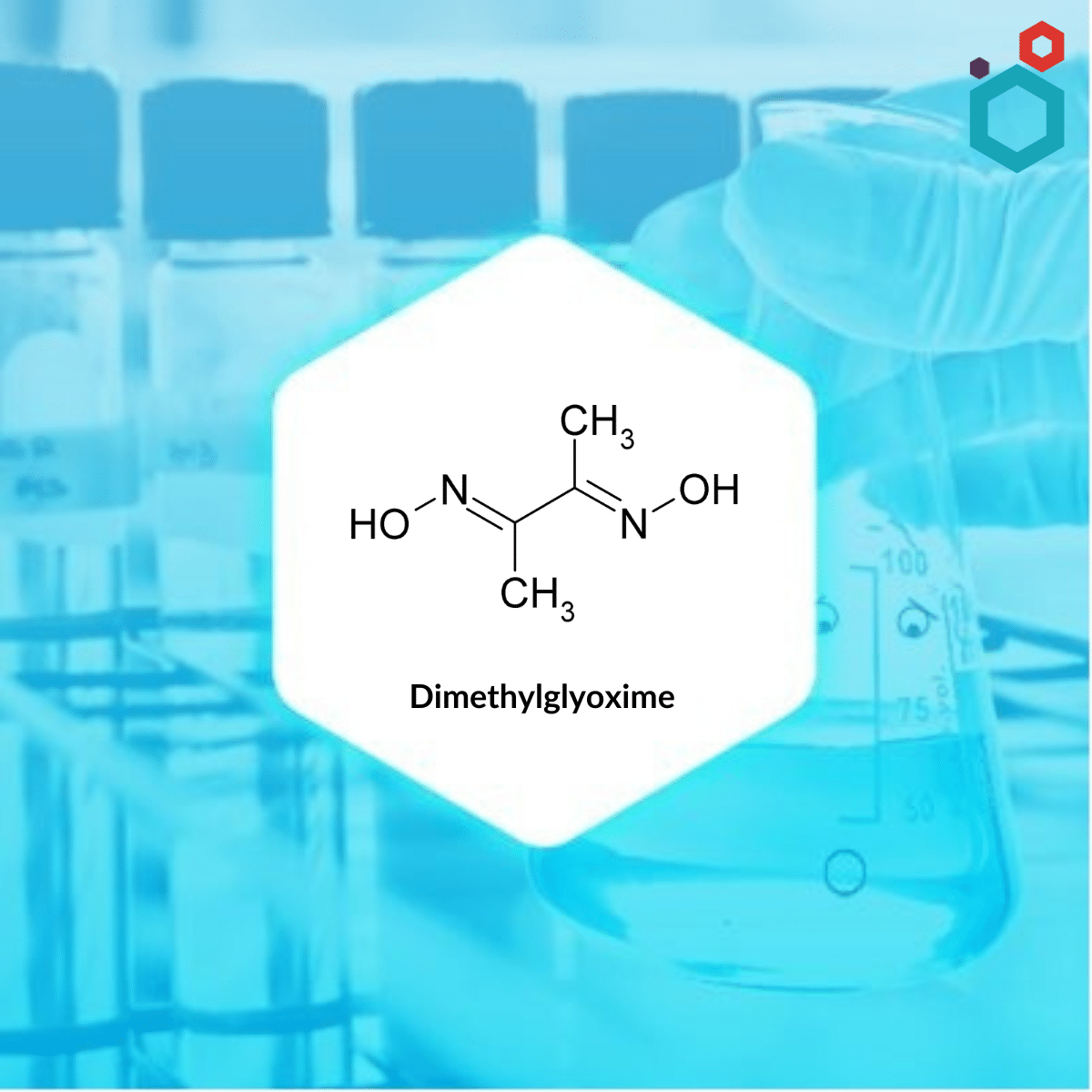
Dimethylglyoxime, also known as DMG, is a chemical compound with the molecular formula C4H8N2O2. It is a diamide i.e., containing two amide functional groups in its structure. The neutral and anionic forms of this substance are denoted by the acronyms dmgH2 and dmgH−, respectively, where H represents hydrogen. It is a well known reagent in analytical chemistry.
| PRODUCT SPECIFICATIONS | |
|---|---|
| Name of Product | Dimethylglyoxime |
| IUPAC Name | N,N′-Dihydroxy-2,3-butanediimine |
| Synonyms | Biacetyl dioxime; Diacetyldioxime; Chugaev's reagent; 2,3-Diisonitrosobutane; Butane-2,3-dioxime |
| CAS No | 95-45-4 |
| Molecular Formula | C4H8N2O2 |
| Molecular Weight | 116.12 g/mol |
| Pubchem CID | 135399895 |
CHEMICAL PROPERTIES
| SR. No | Criteria | Limit/Specification |
|---|---|---|
| 1 | Appearance (form) | Crystalline Solid Powder |
| 2 | Appearance (color) | White to Off-white |
| 3 | Solubility | Soluble 2% in Methanol |
| 4 | Density | 1.37 g/cm3 |
| 5 | Melting point | 238-242°C (dec.) |
| 6 | Sulphated Ash | NMT 0.1% |
| 7 | Assay (Via Nickel complex) | NLT 98.0% |
Uses
Dimethylglyoxime has several uses in analytical chemistry, some of which are as follows-
- Dimethylglyoxime is commonly used to detect the presence of certain metal ions, like nickel, palladium or cobalt. It forms brightly colored precipitates or complexes with these metal ions, which makes it useful for their qualitative and quantitative analysis in various samples.
- It can act as a bidentate ligand in coordination compounds. It forms stable chelates with various metal ions, and these complexes are often used in the study of coordination chemistry, crystallography, and spectroscopy.
- Dimethylglyoxime is sometimes used in organic synthesis, particularly in reactions involving the functionalization of carbonyl compounds or the formation of oximes.
- It is additionally employed in the refinement of precious metals, such as precipitating palladium from palladium chloride solutions.
FAQs
Q. What is dimethylglyoxime used for?
Dimethylglyoxime has many applications in analytical chemistry, which includes as a detecting reagent, a precipitating reagent, and a photometric reagent for various metal ions such as platinum, palladium and nickel.
Q. What are the hazards associated withe use of dimethylglyoxime?
Dimethylglyoxime may cause irritation upon inhalation, ingestion or upon coming in contact with eye/skin. It is also flammable under certain conditions and thus needs to be handled with care.
Q. Is DMG basic or acidic?
Dimethylglyoxime (DMG) is a weak acid.
Buy high purity Dimethylglyoxime from Macsen Laboratories. For buying, send us an enquiry-
