Table of Contents
What is Viloxazine ?
Viloxazine is a non-adrenergic medication belonging to the class of selective norepinephrine reuptake inhibitors (NRI), primarily used for the treatment of Attention deficit hyperactivity disorder (ADHD) in children (aged above 6 years) and adults. Before its discontinuation and subsequent repurposing as an ADHD treatment, it was also marketed for nearly 30 years as an antidepressant for the treatment of depression. Chemically, it is a morpholine derivative and an aromatic ether. It has two stereoisomers, the (S)-(–)-isomer being five times as pharmacologically active as the (R)-(+)-isomer.
Viloxazine is a prescription medicine sold under the brand name Qelbree and formerly as Vivalan and others, available as extended-release capsules. It is a non-stimulant medication, has no known misuse liability and is not a controlled substance.
Chemical Structure & Properties
| PROPERTIES | |
|---|---|
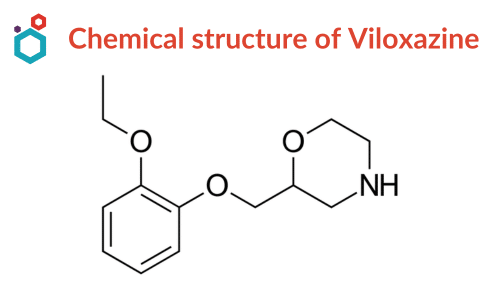
| Name | Viloxazine |
| IUPAC Name | 2-[(2-ethoxyphenoxy)methyl]morpholine |
| Synonyms | Viloxazin; Emovit; Viloxazina; Viloxazinum |
| CAS No | 46817-91-8 |
| Molecular Formula | C13H19NO3 |
| Molecular Weight | 237.29 g/mol |
| Appearance (Form) | Powder |
| Appearance (Color) | White to Beige |
| Solubility | DMSO: 2 mg/mL, clear |
| Melting Point | 178-180°C |
| Pubchem CID | 5666 |
| Pubchem SID | 473127228 |
History
Viloxazine was discovered by scientists at Imperial Chemical Industries and was first described in the scientific literature as early as 1972. The medication was first marketed in 1974 as an immediate-release formulation for the treatment of depression in Europe, however, it was not approved for medical use by the FDA. The FDA granted the medicine orphan status for the treatment of cataplexy and narcolepsy in 1984, with the tentative brand name “Catatrol”. However, for unclear reasons, it was never approved or distributed in the United States for these uses. In 2002, for unknown commercial reasons, it was removed from all marketplaces around the world.
In April 2021, the FDA gave its approval to the use of extended-release formulation of Viloxazine as a treatment for attention deficit hyperactivity disorder (ADHD) based on evidence from several clinical trials conducted at around 59 sites in the United States, having around 1289 participants with ADHD.
Mechanism of action (How does Viloxazine work)
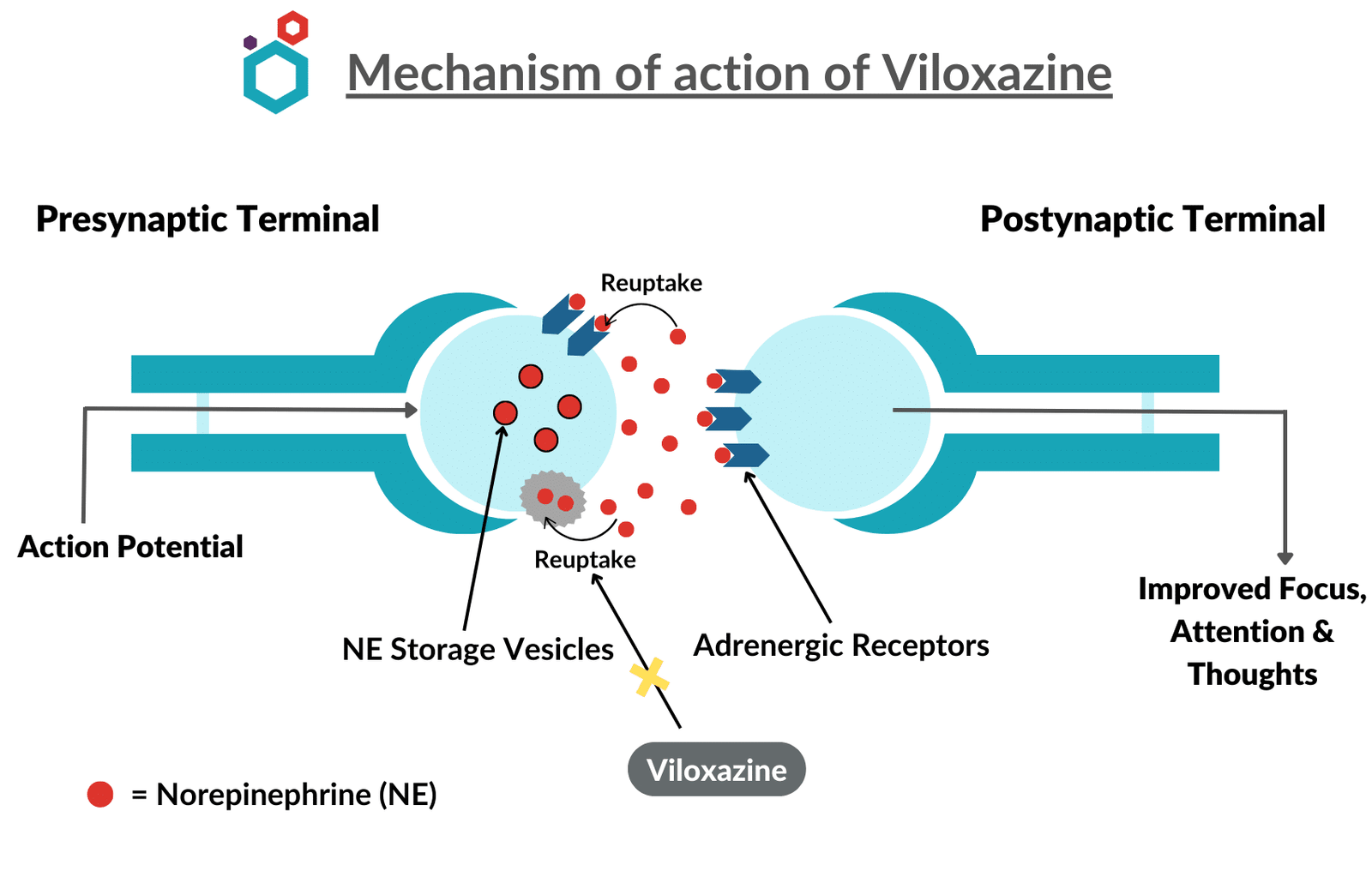
ADHD is a prevalent neurodevelopmental disorder characterized by inattention and hyperactivity in children. Current research suggests that the pathophysiology of ADHD involves an imbalance of neurotransmitters, particularly dopamine (DA) and norepinephrine (NE). These neurotransmitters play a crucial role in the regulation of attention, impulse control, and executive functions. Alterations in the availability, reuptake, or receptor sensitivity of these neurotransmitters are believed to contribute to the symptoms observed in ADHD.
Viloxazine is a medication that belongs to the class of selective norepinephrine reuptake inhibitors (NRI). Its precise mechanism of action is not fully understood, but it is believed to exert its therapeutic effect through modulating the monoaminergic neurotransmitter systems. It inhibits norepinephrine reuptake by binding to the norepinephrine transporter. By blocking the reuptake of norepinephrine, viloxazine increases its concentration in the synaptic cleft, leading to enhanced neurotransmission, which helps in improving focus, attention, and other cognitive functions.
In addition, viloxazine may also have modulatory effects on other neurotransmitter systems, such as dopamine and serotonin, although the magnitude and significance of these effects have not yet been fully determined.
Uses
Use in ADHD
ADHD stands for Attention Deficit Hyperactivity Disorder. It is a neurodevelopmental disorder that typically begins in childhood and often persists into adulthood. ADHD is characterized by a persistent pattern of inattention, hyperactivity, and impulsivity that can significantly impact a person’s functioning and daily life.
Viloxazine is a U.S. Food and Drug Administration (USFDA) approved drug to treat ADHD in children aged 6 to 12 years, adolescents aged 13 to 17 years, and adults. Its extended-release formulation has been studied in clinical trials and has shown efficacy in reducing ADHD symptoms, including inattentiveness, hyperactivity, and impulsivity. It is also approved in the European countries.
Other Uses
- Viloxazine was formerly marketed as an antidepressant for the management of mild to moderate as well as severe Major depressive disorder. The usual dose range for depression was between 100 mg and 400 mg per day, administered typically two to three times per day.
- Viloxazine has been the subject of two randomized, controlled trials for the treatment of nocturnal enuresis (bedwetting) in children, both of those times against imipramine. It was perceived as a less cardiotoxic alternative to imipramine, and to be particularly effective in heavy sleepers.
- It has also been investigated for alcoholism treatment.
- Viloxazine has been shown to suppress cataplexy and unusual sleep-onset REM sleep in patients with narcolepsy.
Dosage
Viloxazine is available in the dosage form of capsules, particularly extended-release capsules, in three strengths – 100 mg, 150 mg and 200 mg. These capsules can even be opened and sprinkled into food for easier administration.
Generally, typical dosing of Viloxazine depends on the age-group to which the individual belongs :
- In adults – The typical starting dose is 200 mg by mouth once daily. The maximum dose is 600 mg/day.
- In children aged 12 to 17 years – The typical starting dose is 200 mg by mouth once daily, followed by weekly 200 mg increments if required (depending upon response and tolerability), not exceeding 400 mg/day.
- In children aged 6 to 11 years – The typical starting dose is 100 mg by mouth once daily, followed by weekly 100 mg increments if required (depending upon response and tolerability), not exceeding 400 mg/day.
The dosage can be modified and may vary from person to person depending on various factors such as any pre-existing conditions related to renal or hepatic impairment, blood pressure, heart rate, etc. One should always consult their healthcare practitioner for periodical re-evaluation and dosage adjustment where the pharmacological treatment is required for extended periods.
Side effects
The side effects associated with the use of Viloxazine can vary from person to person, and not all individuals will experience them. Common side effects reported with the use of viloxazine include:
- Gastrointestinal effects: Dry mouth, nausea, vomiting, abdominal pain (epigastric pain), diarrhea, and loss of appetite.
- Central nervous system effects: Dizziness, headache, insomnia, drowsiness, somnolence, fatigue, tremors, vertigo and sedation.
- Cardiovascular effects: Increased heart rate, palpitations, increased erythrocyte sedimentation, EKG and EEG anomalies and changes in blood pressure.
- Psychiatric effects: Mood swings, anxiety, agitation, irritability, mental confusion and changes in behavior.
- Other rare but potential side effects: Seizures, edema of the lower extremities, suicidal thoughts, mania or hypomania in people with bipolar disorder, etc.
Comparison with Other Compounds
Viloxazine vs Atomoxetine
- Both Viloxazine hydrochloride and Atomoxetine hydrochloride are selective norepinephrine reuptake inhibitors (SNRI), used in capsule form to treat attention-deficit hyperactivity disorder (ADHD), and are marketed under the brand names Qelbree and Strattera respectively.
- Both are non-stimulant oral medications approved by the FDA to treat ADHD in adults and children 6 years of age and older, and share similar mechanisms of action and side effects profile, thus being equally efficacious.
- Atomoxetine is more widely available and approved in various countries as compared to Viloxazine.
- Comparing their capsule dosage form, Strattera is typically taken once or twice a day whereas Qelbree is taken only once a day.
- Another distinction is that Strattera capsules must be consumed unopened, intact and whole. Qelbree can be ingested whole, but the capsule can also be opened and the contents sprinkled onto food for easier administration, being especially helpful for children who may have trouble swallowing pills.
Viloxazine vs Adderall
- Viloxazine is a single active ingredient medication whereas Adderall is a combination medication containing a mixture of dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate.
- Viloxazine is prescribed for the treatment of ADHD whereas Adderall is used for ADHD as well as Narcolepsy.
- Viloxazine is a long-acting, once daily, non-stimulant medicine. On the other hand, Adderall is a short-acting, stimulant medication given once or twice a day.
- Viloxazine is not a controlled substance whereas Adderall is a Schedule II controlled substance with the potential for misuse, drug dependence, and illicit distribution to others.
- Viloxazine intoxication is extremely rare. However, Adderall when taken in higher doses or by individuals without a medical need can produce stimulant effects such as increased energy, euphoria, heightened alertness and a sense of confidence or well-being, that may resemble intoxication.
FAQs
Q. Which potentially serious adverse effect is associated with Viloxazine ?
The common side effects associated with Viloxazine include headache, drowsiness, nausea, vomiting and decreased appetite. While no such potentially serious adverse effect has been reported with the use of Viloxazine, It might cause seizures and may lead to hepatotoxicity rarely in some cases.
Q. Why was Viloxazine withdrawn ?
In 2002, Viloxazine was taken off from markets worldwide for undisclosed commercial and business reasons, unrelated to effectiveness or safety.
Q. Is viloxazine a controlled substance ?
Viloxazine is not a controlled substance and has no abuse potential.
Q. Is viloxazine a stimulant ?
Viloxazine is not classified as a stimulant. It belongs to the class of medications known as selective norepinephrine reuptake inhibitors (NRI) and works by increasing the levels of norepinephrine in the brain by inhibiting its reuptake and help regulate mood and improve symptoms associated with ADHD.
Q. What is the brand name for Viloxazine?
Viloxazine is sold under the brand name ‘Qelbree‘ and is used to treat attention deficit hyperactivity disorder (ADHD) in children and adults.
Q. Is Viloxazine available in the US?
Viloxazine is available in the US. The USFDA granted approval in April 2021 for the use of extended-release viloxazine capsules in the treatment of attention deficit hyperactivity disorder (ADHD) in children and adolescents.
Q. Where to purchase Viloxazine hydrochloride ?
Macsen Labs is a GMP and ISO certified manufacturer and supplier of high-quality Viloxazine hydrochloride as a bulk drug or API. If you want to purchase Viloxazine HCl as a medication, then we suggest that you should contact your nearby pharmacy store with a prescription, subject to that it is approved in your country.
Disclaimer-
The information provided here is based on general knowledge, articles, research publications etc. and we do not claim the authenticity of any of the information provided above. We do not claim or suggest/advise any medical, therapeutic, health or nutritional benefits of Viloxazine. We do not supply or promote our Viloxazine product for the applications which are covered by valid patents and which are not approved by the FDA.