Introduction
Atovaquone is a drug used to manage the mild form of pneumocystis pneumonia, a form of pneumonia caused due to a yeast-like fungus called pneumocystis jirovecii. This form of pneumonia is more common in people with compromised immunity.
Atovaquone Export Insights
Atovaquone API market
Major exporting countries
India has been a leader in the API and finished market of the drug Atovaaquone. India is the major Exporter of this API with around 20 tonnes of average annual exports.
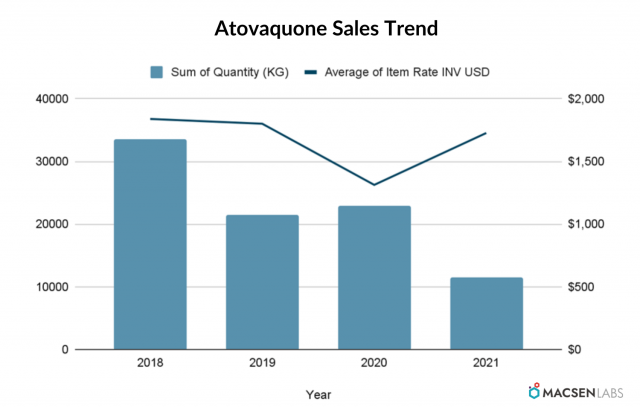
Export from India
- The export quantity has shown fluctuation on YoY basic. It decreased in 2019, then increased in 2020 and again decreased in 2021.
- The average item rate has decreased till 2020 before increasing in 2021.
Three top companies exporting are :
- Divis Laboratories
- Glenmark Pharmaceuticals
- Hetero Drugs
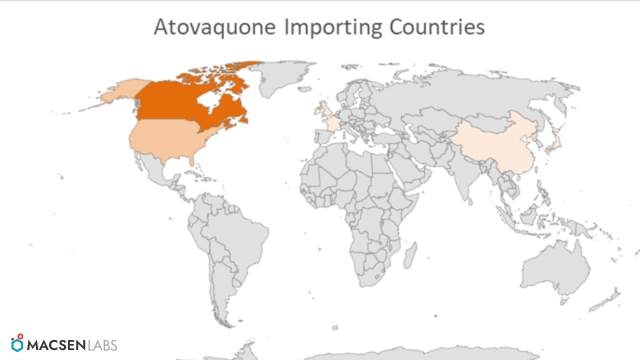
Major importing countries
- Canada and USA are the biggest importing countries of the API from India.
Other Countries Importing are:
- Germany
- Croatia
- United Kingdom
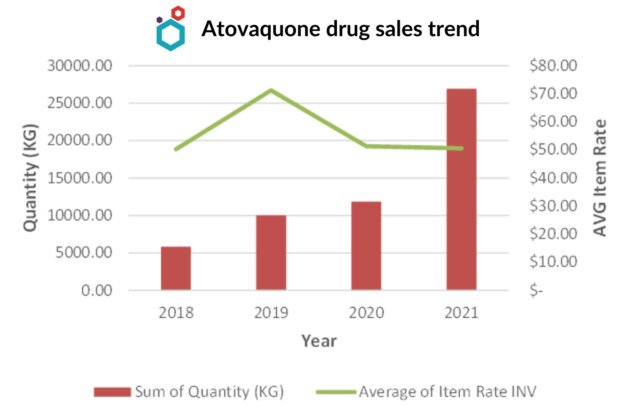
Atovaquone Drug Market
Major Importing Countries
- United States, Hungary, Czech Republic are the major importing countries of Atovaquone drug from India.
Other importing countries include :
- Saudi Arabia
- South Africa
- Germany
This is just a snapshot of the research that we have done on Atovaquone. This is all that the full research report covers:-
- Competitive landscape comprising key countries, and companies.
- Comprehensive Coverage of US and other key markets and other crucial information.
- Complete coverage of both Qualitative and Quantitative Inputs about the drug.
For a negligible price you can buy the full report from us. Drop us
For the entire research report on Atovaquone, send us an enquiry-
Macsen Labs is the supplier of high-quality Atovaquone.
Disclaimer–
The information provided here is based on general knowledge, articles, research publications etc and we do not claim the authenticity of any of the information provided above. We do not claim or suggest/advise any medical, therapeutic, health or nutritional benefits of Atovaquone. We do not supply or promote our Atovaquone product for the applications which are covered by valid patents and which are not approved by the FDA.
